
We invest in therapies that have the power to transform lives, not just markets.
By putting patients first, we believe we can deliver lasting value to everyone involved in bringing our vision to life—from clinicians and physicians to our shareholders.
By reimagining what’s possible in medicine we’re unlocking tomorrow —where science doesn’t just treat diseases, but restores lives.
Business model
Scaling Innovation
We are dedicated to exploring and discovering effective cell therapies worldwide. By developing these innovations and bringing them to the global market, we commit to improving patients' health and quality of life (QOL) through the most cutting-edge treatments possible.

Features
Target Indication Characteristics
Currently, Innovacell is engaged in the development and commercialization of three autologous cell therapy candidates targeting incontinence areas, specifically urge & stress fecal incontinence and urinary incontinence.
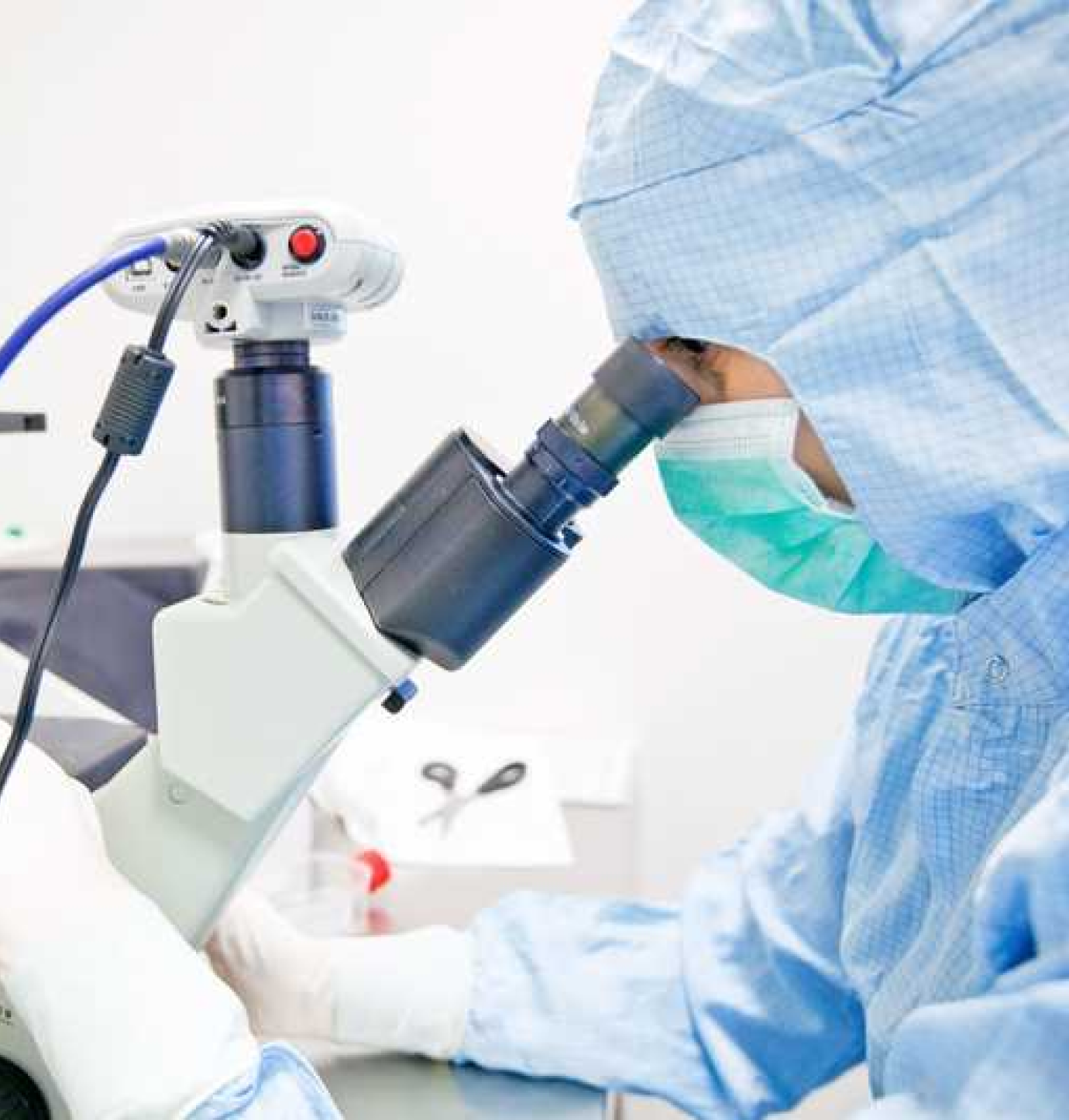
Engineering
Manufacturing the Future
Innovacell’s manufacturing technology has been developed at our GMP manufacturing facility in Austria. This GMP manufacturing facility was the first to obtain the "Manufacturing Certification for Specific Cell Processing Products" as a facility located outside Asia by the Ministry of Health, Labor, and Welfare of Japan.

Pipeline
Translating Research into Healthcare
We are currently engaged in the development and commercialization of three cell therapy candidates targeting both fecal and urinary incontinence.

Company Information

Careers
While we are always looking for talented, driven individuals to join our team there are currently no open positions.
Please check back at a later date.
Thank You.

